News from the Group
Stepping up against mpox: Strengthening Guinea's diagnostics and sequencing capacity
After the declaration of the Public Health Emergency of International Concern (PHEIC) for mpox on 14 August 2024, our team traveled to Guinea a few days later to support the implementation of sequencing capacity for mpox at the laboratory for viral hemorrhagic fevers (VHFs) in Conakry (CRV-LFHVG). Supported by our GHPP program CELESTA, a one-week training was offered to deep dive into sequencing data analysis and enhance the bioinformatics skills of the local team. A refresher training in mpox virus diagnostics, which had been setup back in 2022, was also performed. As a result of our joint preparedness efforts, Guinea's first-ever confirmed case of mpox was reported in September 2024! Two laboratories were involved in this early detection and characterization, the laboratory based in Gueckédou, LFHV-GKD, and the CRV-LFHVG from Conakry.
Building on this achievement, our team performed a new training in October 2024 to further improve the diagnostics capacity and readiness for mpox virus detection. Previously, diagnostics efforts relied on two tests to detect a broad range of zoonotic orthopoxviruses, including mpox virus. However, the need for mpox virus-specificity prompted the implementation of a new real-time PCR. This upgrade improves both diagnostics accuracy and laboratory standards of the three laboratories located in Conakry, Gueckédou and N’Zérékoré. During this one-week training, 16 participants from the capital town and forest region learned about the virology, epidemiology, clinical presentation and global spread of mpox while also becoming proficient in hands-on practice. Soft skills development were another key component of our program with trainees collaborating in creating poster presentations, enhancing their competencies scientific communication and teamwork! The training provided the opportunity to engage into a new phase of the program with the Training of Trainers (ToTs). One ToT from LFHV-GKD further conducted the same training curriculum in the laboratories of N’Zérékoré and Gueckédou training thus another 11 staff in November 2024. This was a pivotal training to guarantee harmonized diagnostics processes setup in the whole laboratory network.
These initiatives and successes highlight our joint commitment to support Guinea’s readiness to detect and respond to future health challenges promptly. Collaboration and preparedness remain at the heart of these efforts as we work together to safeguard public health.




Strengthening Lassa Fever Testing: Capacités Renforcées à Parakou, Bénin
Lassa Fever (LF), a disease listed as a priority by the World Health Organization (WHO) R&D Blueprint, continues to pose significant health risks across West Africa. Urgent research, particularly in the development of therapeutics and vaccines, is critical. In this context, the INTEGRATE consortium is dedicated to preparing clinical sites for upcoming trials of LF therapeutic candidates, with a special focus on capacity-building initiatives. As part of this effort, our team, in collaboration with ALIMA (The Alliance for International Medical Action) embarked on a site preparedness programme (SPP) funded by PANTHER (PANdemic preparedness plaTform for Health and Emerging infectious Response) aimed at enhancing laboratory capacities. The programme consisted of a series of online trainings on clinical research and on-site activities in Parakou, Borgou department, Benin through the implementation of RT-PCR diagnostics for Lassa virus (LASV).
The core of our work in Benin centered on strengthening the laboratory workforce. We provided comprehensive training for the handling and testing of Risk Group 4 pathogen samples, such as those of the Lassa virus, ensuring that the lab technicians could safely and effectively manage samples. Additionally, we improved the lab’s workflow by implementing critical procedures for safe sample reception and handling, process documentation, and adopting Good Clinical Practice (GCP) and Good Clinical Laboratory Practice (GCLP) standards.
The programme offered a blended learning experience, combining remote and in-person formats to maximize flexibility and engagement. Five laboratory scientists from the Centre d’Informations de Prospective et de Conseils sur les IST/VIH Sida (CIPEC) in Borgou/Alibori were trained, marking the successful implementation of Lassa virus RT-PCR diagnostics in Northern Benin in November 2023. The trainees not only gained hands-on experience with molecular diagnostics but also attended lectures on clinical research, quality management, and data handling.
Our work in Benin underscores the importance of both national and international collaboration in addressing public health challenges. By building local expertise, we aim to close gaps in logistics, infrastructure, and technical knowledge, ensuring that laboratories in resource-limited settings are prepared for outbreaks and be equal partners for research. The significance of the SPP was highlighted in September 2024 at the World One Health Congress in Cape Town, South Africa. Here, we shared the impact of the collaborative efforts in Benin, showcasing the outcomes of our capacity-strengthening programme and its role in improving Lassa fever diagnosis and treatment efforts across West Africa.
As we move forward, the sustained investment in capacity-building efforts will remain crucial. By continuing to strengthen the research and diagnostic capabilities of laboratories in Benin and beyond, we hope to improve disease surveillance, response, and preparedness for future public health emergencies.
Further reading:



“WONTANARA!”: “Nous sommes ensemble” in Guinea!
Our laboratory support to Guinea continues thanks to the GHPP programs CELESTA and AfroLabNet 2.0 as “We are together”!
Our team has developed an exciting and adaptable training portfolio spanning from real-time RT-PCR diagnostics to serology and up to the complexity of metagenomic sequencing! During a month-long visit in May 2024, we delivered such training at our partner laboratories for viral hemorrhagic fevers (VHFs) in Conakry (CRV-LFHVG), Gueckédou (LFHV-GKD) and N’Zérékoré (HRNZE-LFHV). The three laboratories support the in-country surveillance of infectious viral diseases, and regularly reinforcing such capacity is key. This time, we offered new learning approaches to strongly engage the staff in our trainings, as observing soft white DNA extracts from bananas! Also, two staff from the Gueckédou laboratory joined a training in Conakry to unravel the secrets of nanopore sequencing and phylogenetic analysis. Beyond knowledge increase of staff, it further strengthened the network between these three key laboratories which are pillars of Guinea’s surveillance system of viral hemorrhagic fevers.
Besides diagnostic capacities, our joint research efforts aim at understanding the circulation of relevant viral diseases in communities. Thanks to the serology platforms established in the laboratories of Gueckédou and N’Zérékoré, crucial studies are ongoing. The “REALISE” study, led by Fara Raymond Koundouno, will soon give us more insights into the seroprevalence of Lassa fever, Marburg virus disease and COVID-19 in communities of the Gueckédou prefecture, whereas Youssouf Sidibé, leading another study, will finally give us soon answers on Lassa fever seroprevalence in another prefecture, N’Zérékoré. We expect compelling outcomes from these researches that will guide health systems of Guinea and other affected countries.
In partnership with WHO Geneva and WHO Guinea Country Office, the preparedness and readiness level of the three laboratories has been bolstered by the provision of diagnostics reagents for the detection of Ebola virus, Marburg virus, Lassa virus, Yellow fever virus and Dengue virus, allowing for full diagnostic activities in the coming months.



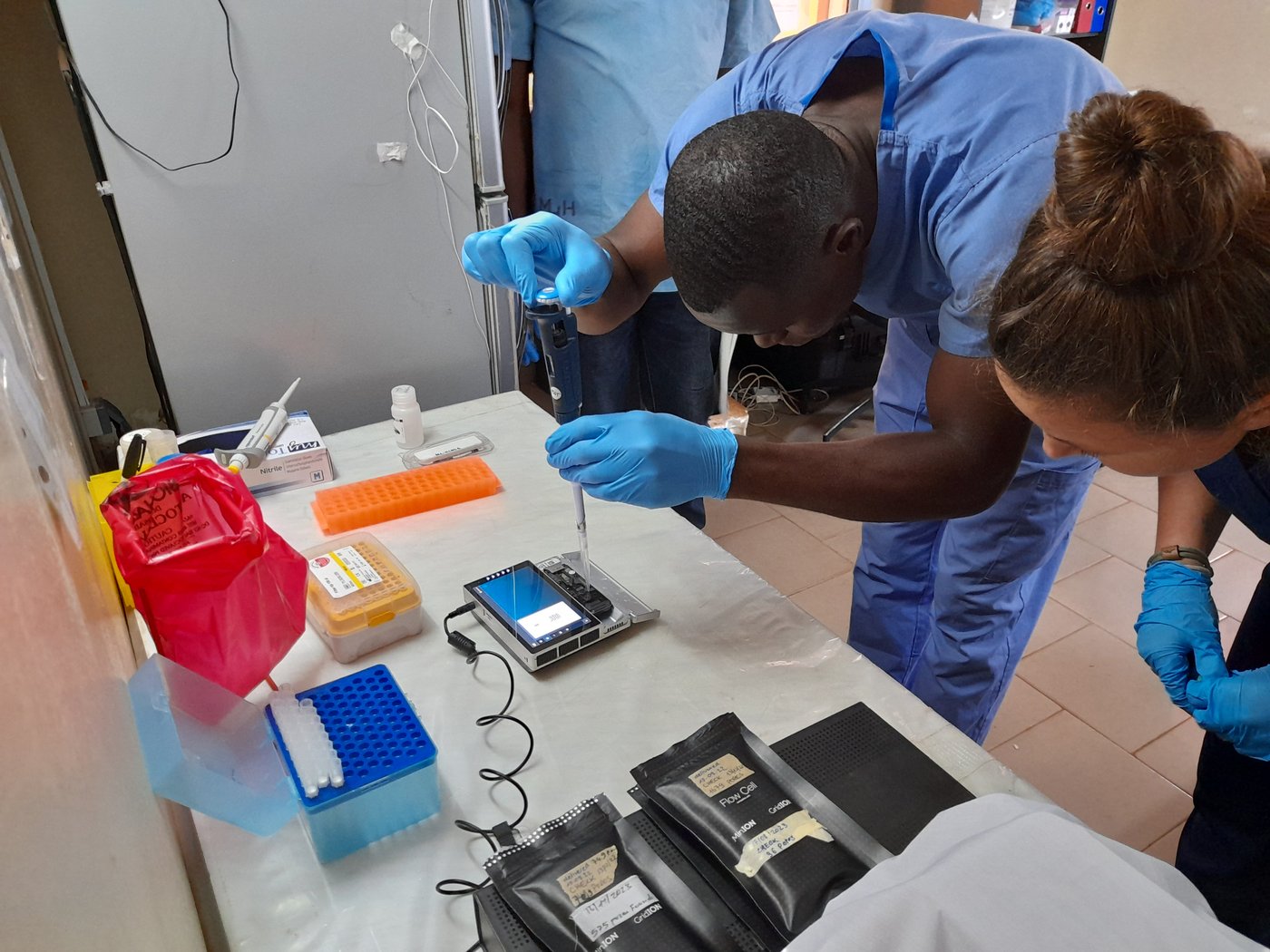
Embarking on a New Era: Enhanced Sequencing Capacity at Irrua Specialist Teaching Hospital (ISTH), Nigeria
As the dry season unfolds in 2024, clinicians, healthcare professionals, and laboratory experts at the Irrua Specialist Teaching Hospital (ISTH) in Nigeria brace themselves for the onset of yet another Lassa Fever outbreak. Lassa fever, an acute viral hemorrhagic illness caused by Lassa virus, remains a persistent challenge in West Africa, particularly in Nigeria. Swift and accurate detection, coupled with real-time monitoring of circulating viruses, are paramount to reinforce the efforts of the Ministry of Health (MoH), the Nigeria Center for Disease Control (NCDC), and the World Health Organization (WHO) in controlling the outbreak.
In line with our commitment to collaborative activities, in February 2024 a team from BNITM embarked on a journey to ISTH as part of our Global Health Protection Program (GHPP) CELESTA which aims to fortify local genomic surveillance capabilities. Our mission? To expand the pre-existing SARS-CoV-2 nanopore sequencing capacity at the Institute of Viral and Emergent Pathogens Control and Research (IVEPCR) of ISTH. This expansion is geared towards empowering IVEPCR to establish a comprehensive genomic surveillance framework, employing the metagenomics approach on the MinION sequencing platform, to track not only Lassa virus but also any emerging RNA pathogens.
Following an intensive two-week training, the dedicated sequencing team at ISTH mastered the hands-on sequencing practice and the implementation of cutting-edge bioinformatic tools for sequencing data and phylogenetic analyses. Equipped with these skills, the local team now stands poised to independently operate the sequencing unit onsite. This capability enables real-time characterization of circulating Lassa viruses and other RNA pathogens, empowering ISTH and the broader Nigerian public health response in combating the ongoing Lassa fever outbreak.
The inauguration of this enhanced sequencing capacity marks a significant milestone, underscoring our collective dedication to supporting surveillance efforts. Moreover, it serves as a testament to the commitment of both BNITM and ISTH to collaborative research (for further details, see here. With the support of GHPP-CELESTA, BNITM will continue to provide ongoing support to the ISTH sequencing team through future training initiatives, as well as theoretical and technical assistance.


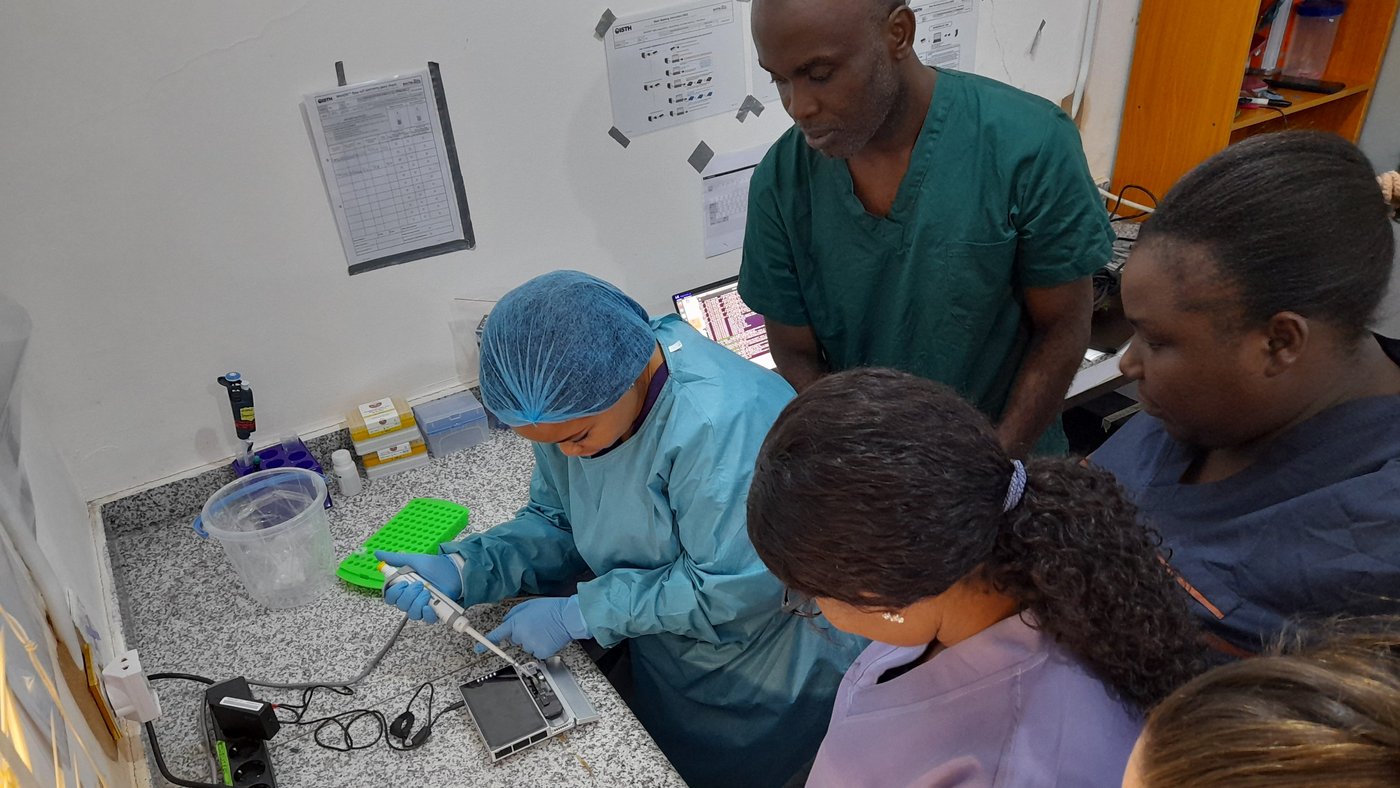
Postcard from Forest Guinea: setup of new laboratory capacities to strengthen the surveillance of viral hemorrhagic fevers
As part of our GHPP-funded programs Celesta and AfroLabNet 2.0 we traveled to Forest Guinea in November 2023 to expand the serological and genomic surveillance capacities of two collaborating laboratories, respectively the Laboratoire des Fièvres Hémorragiques Virales de l’Hôpital Régional de N’Zérékoré (LFHV-HRNZE) in N’Zérékoré and the Laboratoire des Fièvres Hémorragiques Virales de Gueckédou (LFHV-GKD) in Gueckédou.
Forest Guinea has faced several epidemics of viral hemorrhagic fevers (VHFs) in previous years such as Ebola and Marburg virus diseases, as well as Lassa fever. In support of Guinea’s laboratory epidemic preparedness, our programs focus on addressing laboratory gaps by implementing new capacities to complement the already established VHF diagnostics platforms of LFVH-HNRZE and -GKD.
Following a 1-week training in N’Zérékoré with four participants, our team successfully completed the setup of a new serology unit at LFHV-HRNZE. The training specifically focused on the detection of immunoglobulin M and G antibodies against Lassa virus. The latter is currently used for operational research on VHFs.
In addition we held a 2-week introductory training on nanopore sequencing (Oxford Nanopore Technologies) applied to RNA-virus genomic surveillance at LFHV-GKD in Gueckédou. The six staff attending it were primed to basic hands-on and related bioinformatics analysis. This was a milestone achievement in preparation of the VHF genomic surveillance unit to be implemented in 2024-2025 at LFHV-GKD.
These trainings were key in allowing the two laboratories to expand their portfolio of services for the benefit of the Guinean public health surveillance system. BNITM, through GHPP, will continue to support Guinea with other trainings in the coming months and, remotely, with theoretical and technical assistance.



The EMLab participates in the medical EU-MODEX in Çanakkale, Türkiye (18-22 Sept. 2023)
The European Mobile Laboratory (EMLab), managed through the Virology Department and Mobile Laboratory group at BNITM, successfully participated in the medical European Union Module Exercise (EU-MODEX1) for civil protection capacities under the EU Civil Protection Mechanism (UCPM2) in Çanakkale, Türkiye (18-22 September 2023). This was part of the final step towards certification of the EMLab in the EU Civil Protection Pool (ECPP3) as a mobile laboratory capacity from Germany.
The field exercise simulated a medical emergency in the aftermath of an earthquake in Çanakkale, Türkiye, requiring the deployment of different ECPP capacities including emergency medical teams (EMTs), ambulance services, air medical evacuation, technical support assistance teams (TAST), as well as mobile labs. The EMLab team worked closely with other response capacities and provided support to EMTs and local hospitals with basic point of care tests, molecular diagnostics and genomic surveillance.
Check out the official event video from the MODEX press team here:
https://youtu.be/Wfoi_n6imSs?feature=shared
Interoperability and coordination in emergency situation are one of the key components in such MODEX and EMLab, in addition to collaborating closely with various response capacities, also interacted with stakeholders from other relevant organizations including the European Commission (EC), World Health Organization (WHO), United Nations (UN), Turkish Ministry of Health and Turkish Ministry for Disaster Management and Civil Protection (AFAD).
The EMLab team comprised 12 experts from the BNITM and from other institutions part of the EMLab network including the Robert Koch Institute (Berlin, Germany), Scientific Solutions (Stockholm, Sweden) and University of Pécs (Pécs, Hungary).





First step in the expansion of genomic surveillance capacity in Guinea and Nigeria
To strengthen the pre-existing sequencing capacities of our GHPP-partner laboratories in Conakry (Guinea) and Irrua (Nigeria), a joint two-week training on metagenomic sequencing was held in Conakry, Guinea, in June 2023.
Following the successful establishment of two SARS-CoV-2 field sequencing laboratories at the Laboratoire des Fièvres Hemorragiques Virales en Guinée (CRV-LFHVG) in Guinea in 2021, and at the Irrua Specialist Teaching Hospital (ISTH) in Nigeria in 2022, the Outbreak Preparedness and Response (OPR) BNITM team continues its long-term program of sequencing capacity reinforcement in our partner laboratories in West Africa. Our project CELESTA, which is part of the new program phase of GHPP (2023-2025), will expand the genomic surveillance capacity for RNA viruses to support the national surveillance system against future epidemics and pandemics.
Taking advantage of the previously established Nigeria-Guinea cooperation, a team from BNITM and four lab scientists from ISTH traveled to CRV-LFHVG in Conakry in June 2023 to conduct a two-week training course. Four laboratory staff from CRV-LFHVG also participated in the training. The main goal was to train local sequencing staff on a new next generation sequencing workflow for metagenomic sequencing, from sample preparation to in-country analysis. During the training the local staff was also introduced to general concepts of virus phylogeny and new bioinformatic analysis tools.





![[Translate to English:] Logo_Alexander_von_Humboldt_Stiftung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Alexander_von_Humboldt_Stiftung.png)
![Logo BMBF [Translate to English:] Logo Bundesministerium fuer Bildung und Forschung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Bundesministerium_fuer_Bildung_und_Forschung.png)
![Logo BMG [Translate to English:] Logo Bundesministerium Fuer Gesundheit](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Bundesministerium_Fuer_Gesundheit.png)
![Logo Centre for Structural Systems Biology (CSSB) [Translate to English:] Logo CSSB](/fileadmin/media/Das_Institut/Kooperationen/CSSB_Logo_01.png)
![Logo Deutsche Forschungsgemeinschaft (DFG) [Translate to English:] Logo DFG](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_DFG.png)
![Logo Deutsches Zentrum für Infektionsforschung (DZIF) [Translate to English:] Logo DZIF](/fileadmin/media/Das_Institut/Kooperationen/Logo_DZIF_01.png)
![[Translate to English:] Logo Emerge](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Emerge.png)
![[Translate to English:] Logo European Virus Archive goes global](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_European_Virus_Archive_goes_global.png)
![Logo Federal Foreign Office [Translate to English:] Logo Federal Foreign Office](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Federal_Foreign_Office.png)
![Logo GHPP [Translate to English:] Logo GHP](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/GHPP-Logo-RGB.png)
![[Translate to English:] Logo GPPEBHS](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_GPPEBHS.png)
![[Translate to English:] Logo iNext](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_iNext.png)
![Logo Jürgen Manchot Stiftung [Translate to English:] Logo Jürgen Manchot Stiftung](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_Juergen_Manchot_Stiftung.png)
![Logo LCI [Translate to English:] Logo LCI](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_LCI.png)
![Logo der Leibniz Gemeinschaft [Translate to English:] Logo Leibniz Gemeinschaft](/fileadmin/media/Das_Institut/Kooperationen/Logo_Leibniz_Gemeinschaft.png)
![[Translate to English:] Logo LFF Hamburg](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_LFF_Hamburg.jpg)
![[Translate to English:] Logo Pandora](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/Logo_PANDORA.png)
![[Translate to English:] [Translate to English:] Logo der Drugs for Neglected Diseases initiative, bestehend den aus drei orangefarbenen Großbuchstaben DND und einem kleinen i mit schwarzem Punkt.](/fileadmin/media/Allgemeines_und_Platzhalter/Logo/DNDi_Logo_EN_Full_Colour_margins.jpg)





